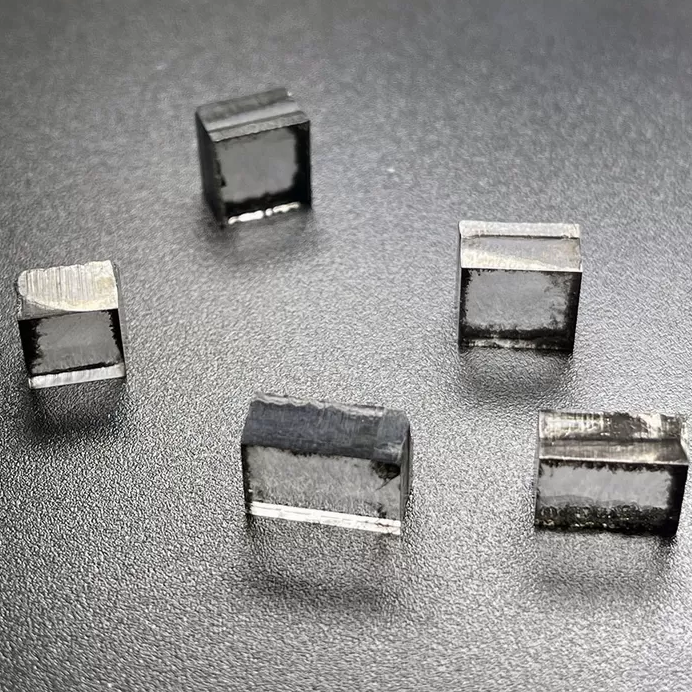
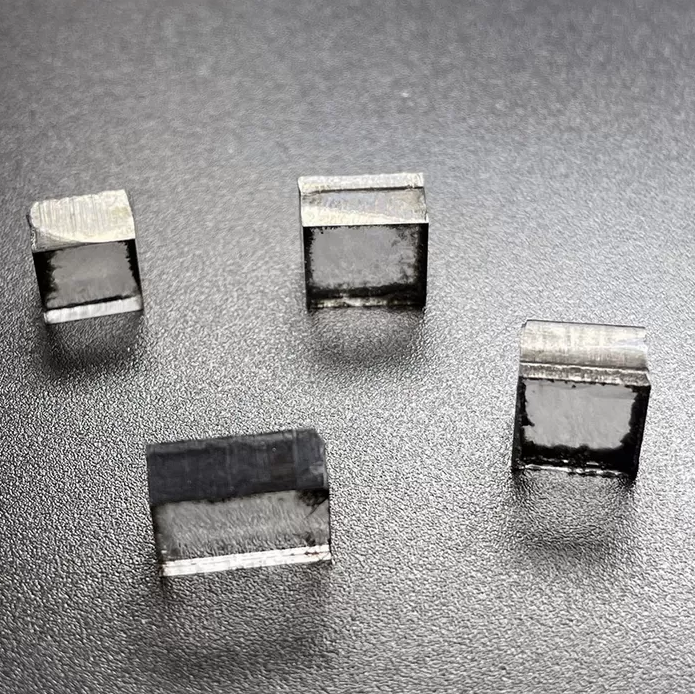
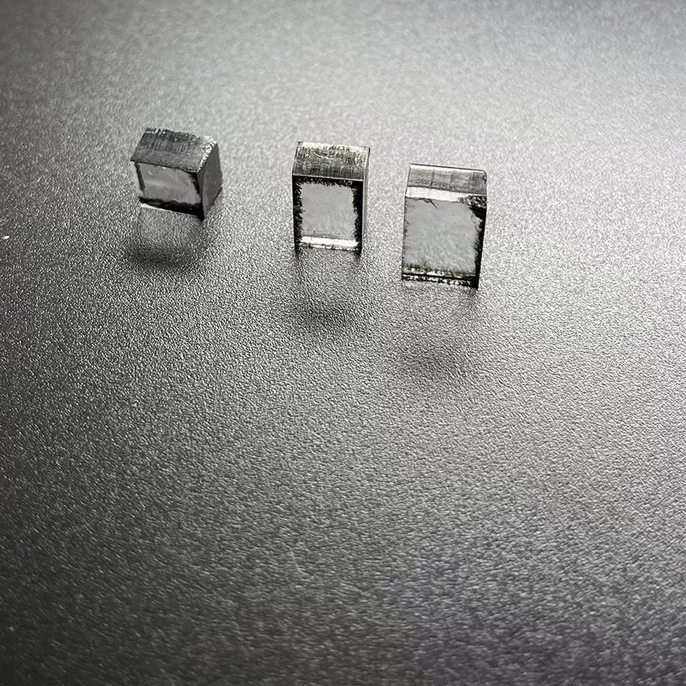
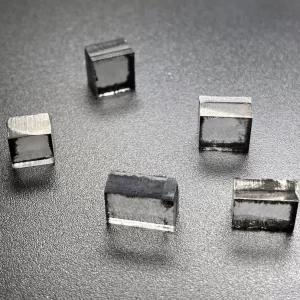
5mm To 7mm DEF Color Rough CVD Diamond Rectangular Shape
CVD Lab Grown Diamonds Description
The laboratory-grown diamonds are grown in the laboratory, they are composed of carbon atoms, have the same physical properties and chemical composition as natural diamonds, and have fewer impurities and higher purity than natural diamonds. And it’s cheaper.
There are two techniques for growing diamond in the laboratory, one is the high temperature and high pressure method. This method is the most widely used, but this process will use a catalyst to mix the diamond with impurities, so most diamonds are used in industrial fields. The other is chemical vapor deposition. This method can obtain high-purity diamond without a catalyst.
What is the essential difference between natural diamonds and cultivated diamonds, mainly in the following points:
1. Formation time
Natural diamonds are formed under high temperature and high pressure due to geological reasons, and it takes tens of thousands or even tens of millions of years to produce. Cultivated diamonds are cultivated in about one to three weeks.
2. Crystal shape
The formation temperature of natural diamonds and laboratory-grown diamonds is almost the same, but natural diamonds will grow into octahedral crystals, while lab diamonds can grow into crystals that contain both octahedrons and cubes.
3. Color
Natural diamonds are colorless, light yellow and other colors. At present, better quality lab diamonds can also achieve colorless or light brown, which is almost unrecognizable to the naked eye.
Tidbits about lab-grown diamonds
Rough CVD Diamond Rectangular Shape
The physical properties of a CVD diamond are determined by the orientation of the grains of the diamond. This article will explain the properties of CVD diamonds and their differences from natural diamonds. You will also learn how to choose the best diamond for your jewelry needs. Whether you are looking for a single diamond or a wedding band, you will find that the rectangular shape is the best choice. Here are some benefits of this shape.
Orientation dependence of diamond grains in dressing tools
In dressing tools, the orientation of rough CVD diamond grains is partially dependent on the type of diamond. NDs and MCDs are monocrystalline, and they are less sensitive to wear. However, CVD diamond is becoming a popular choice for stationary dressing tools, due to its high hardness and quasi-isotropic behavior. The anisotropy of the small monocrystals balances out the effect of the larger ones. PCD rods and logs do not have an oriented preference for a direction.
Diamond has impressive physical properties and is used in a wide range of industrial production processes. Its extreme hardness makes it suitable for abrasive jobs, including the machining of metals, carbides, and glass. Because of its high thermal conductivity, diamond has been used in microwave and telecommunication equipment. Some industries no longer use diamonds as the material of choice, so synthetic versions are used instead.
Moreover, abrasive concentration represents the number of rough CVD diamond grains that are in contact with a workpiece, and affects the load distribution. The higher the concentration, the lower the effective load per grain. Using a mixture of different abrasive materials, such as carbon, can result in production of type IIa diamond grains. While this is less common, the nitrogen present in the rough CVD diamond grains is bound to the getter and bound to the grain.
These characteristics of diamond are responsible for its incredible wear properties. Their high atomic density and rigid lattice prevents a large number of foreign atoms from entering the diamond. However, diamond has small substitutional defects that act as electron donors or acceptors. Its composition contains a wide variety of non-carbon atoms including Si, P, and S. The latter two atoms are responsible for the diamond’s high hardness.
The failure mode in the natural diamond is cleavage on the 111 plane. In Houwman’s 2003 study, he measured the strength of 14 CVD single crystal plates and 14 natural type IIa diamond plates with a surface roughness of one micrometer. The CVD single-crystal plates measured up to 5.1 GPa, while the natural type IIa samples reached 4.5 GPa.
The orientation dependence of rough CVD diamond grains in dressing devices is an important issue. It is an important consideration when choosing a diamond dressing tool because it will greatly affect the cutting and polishing results. The orientation-dependent nature of CVD diamond grain size has led to the increased popularity of the diamond in these tools. Further research is needed to optimize this tool for different applications. In the meantime, CVD diamonds are a viable choice for many applications.
The natural diamond resources are limited and concentrated in a small number of locations. However, synthesis of diamonds has made diamond more accessible, and the prices for producing the stone have decreased significantly. Due to competition from emerging economies, diamond synthesis is now available for less than $400/kg, and diamonds are a valuable investment. The price of diamond has fallen to the lowest level since the invention of diamond synthesis.
Physical properties of CVD diamonds
The physical properties of rough CVD diamonds are impressive. These diamonds have high thermal conductivity at 300 K, high resistivity, and a breakdown voltage of 107 volts per meter. These properties make diamonds very desirable materials for many applications. Read on to learn about diamonds’ physical properties and how they are produced. We’ll also discuss the processes of creating diamonds using low-pressure CVD methods.
Chemical vapor deposition, or CVD, is a process that deposits diamonds in a vacuum chamber. A carbon-containing gas is passed over diamond seeds in a vacuum chamber, which is heated to more than 1,800 degrees. As carbon crystallizes on the diamond seed, it grows to a rectangular shape. Diamonds grown this way have different gemological properties from HPHT-grown diamonds.
The physical properties of a CVD diamond vary considerably from its natural counterpart. Diamonds have a perfect rectangular shape, but may have a frosted surface. This occurs when overheating or mounting repairs have resulted in damage to the diamond’s surface. The carat is the standard weight unit of gemstones. The physical properties of CVD diamonds include colour, clarity, and cut. The clarity grade will determine the size and color of inclusions when viewed at a 10x magnification.
Another difference between natural and lab grown diamonds is their cut. Natural diamonds have many benefits, including their fire and brilliance. When it comes to choosing the shape, the decision is up to the gem cutter. They need to decide which shape is best for the stone to optimize its fire and brilliance. If a diamond has flaws, rounding it will help hide them and maximize the beauty of the stone.
Lab-grown diamonds are a hybrid of natural and synthetic diamonds. In fact, the latter is more expensive than the former, resulting in higher price. They are created under extreme conditions and manipulated using a powerful vise. They mimic the chemical and physical properties of a natural diamond. They are more durable and are harder. Some diamonds are even created in the shape of a heart. They may be the hardest on earth.
A round diamond is the most common shape for engagement rings and other pieces of jewelry. This shape is ideal for maximizing a diamond’s brilliance and brightness. It can be faceted in a variety of shapes, including oval, pear, and princess. In addition to the traditional round diamond cut, a square-shaped diamond is another popular shape. Round diamonds are more expensive than their round counterparts, which are ideal for women who enjoy a trendy lifestyle.
The physical properties of a round-shaped diamond are largely governed by the symmetry of the crystals. Because diamonds are an isotropic material, its optical properties are the same in all directions. If you’re looking for a round diamond, consider a rock with yellow or blue igneous diamonds. There are also some igneous diamond-bearing rocks with magnesium content. Laser-shaping techniques enable diamonds to be cut across growth planes and to form odd-shaped crystals.
Characteristics of natural diamonds
Characteristics of rough CVD diamonds are closely related to the atomic structure of the final product. During diamond growth, graphitic sheets form at the interfaces between diamond and graphite, and then shrink to a closed, curved structure. The process also creates nanotubes, which can be stacked to form MS (molybdenum disulfide).
The synthesis process of diamonds is driven by kinetics, not thermodynamics. In general, the CVD process starts with a small amount of carbon (around five percent) and an excess of hydrogen. The hydrogen can be heated to atomic levels or to molecular hydrogen temperatures. Three common methods are used to heat molecular hydrogen, microwave plasma, and DC arc. Molecular hydrogen is then exposed to the high-energy electrons of a DC arc.
The weak point of chemical vapor-deposited diamond is that it exhibits a certain degree of preferred crystallographic orientation. This feature limits its use as an abrasive material. It is promising, however, as a tool for needle-shaped rods and dressing tools. However, it is more difficult to wire-cut and is more prone to wetting than polycrystalline diamonds. This makes reproducibility of results difficult.
Thermal conductivity is another important characteristic of CVD diamond. The thermal conductivity of 2000 W m-1 K-1 is far greater than that of copper. Lower grades of CVD diamonds have significantly lower thermal conductivity. By contrast, higher grades of diamond have better thermal and mechanical properties. These properties are critical for applications requiring precision and durability, ranging from jewelry to high-end semiconductor devices. The properties of CVD diamonds are highly influenced by their impurity content and grain structure. Generally, the finer the grained diamond is, the greater its thermal conductivity.
The chemical properties of diamond are important for electroanalysis and other applications. The diamond’s sp2 carbon structure allows it to be driven at higher potentials, thereby enabling the creation of electrodes with a wider range of potentials. In addition, diamond has a similar potential window to glassy carbon. The diamonds with sp2 carbon have a similar potential window to glassy carbon and highly oriented pyrolitic graphite. However, they may also disintegrate under large positive voltages.
Interstitial point defects are rare in CVD diamonds. Because diamond is so rigid and has a high atomic density, it is difficult to introduce foreign atoms into the crystal structure. However, small substitutional defects are possible, and these defects serve as both electron donors and acceptors. The CVD diamonds with non-carbon atoms include Si, P, and S. They are very difficult to identify, but the characteristics of a rough diamond can be determined by examining the structure of the rough diamond.
Natural diamonds are also extremely costly, and the size of a natural diamond is limited. CVD diamonds, on the other hand, are far more affordable than their natural counterparts. They also don’t support conflict and leave almost no mineral waste. These characteristics make CVD diamonds the better choice when considering which type of diamond you’d like to buy. Moreover, CVD diamonds are still extremely valuable, so consumers should not be put off by the cost difference.
5mm To 7mm DEF Color Rough CVD Diamond Rectangular Shape