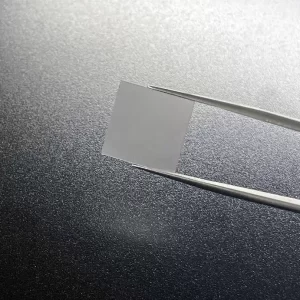
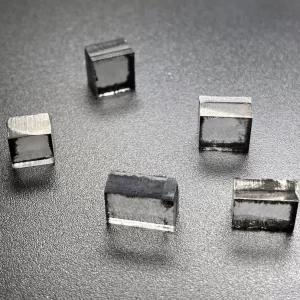
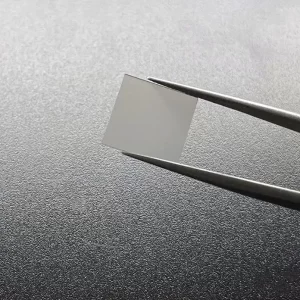
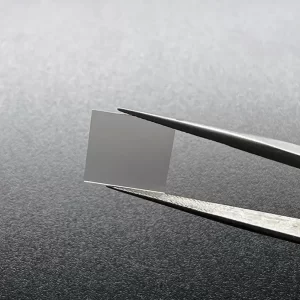
8mm*8mm CVD Single Crystal Diamonds Lab Created Light Brown
8mm*8mm Square Shape Optical Grade CVD Single Crystal Diamonds
CVD Single Crystal Diamonds Description
CVD diamond is made at low pressure. The mixture of carbon containing gas and hydrogen is excited to decompose at high temperature and pressure lower than the standard atmospheric pressure to form active diamond carbon atoms, which are then deposited on the substrate to form polycrystalline diamond (or single or quasi single crystal diamond is deposited under controlled deposition conditions). Because CVD diamond does not contain any metal catalyst, its thermal stability is close to that of natural diamond.
The hardness, wear resistance, thermal conductivity and thermal stability of CVD diamond materials are much better than those of PCD materials, which can be comparable with single crystal diamond. Therefore, CVD diamond materials can achieve efficient processing of high hardness materials and difficult to machine materials, such as glass fiber reinforced plastics. It allows higher processing speed and shows longer service life. With the improvement of industrial processing technology and efficiency in the world, it will replace the widely used PCD blade material in a large range.
CVD diamond material has excellent surface finish and low friction coefficient after polishing, which can greatly reduce the cutting force and friction heat generated during cutting. It has excellent anti burr performance, and the machining finish is close to that of single crystal diamond tool.
Surface: As Grown Or Laser Cut
Thickness: 0.5mm
Edge: Laser Cut or Polished
Size: 8*8mm, can customize
Application: For Making Tools
Crystal Orientation: 100 110 111
CVD Single Crystal Diamonds Lab Created Light Brown
In CVD diamonds, the process begins by depositing carbon onto the diamond seed, which is a diamond-like piece of material, silicon or molybdenum, or cemented carbide. The substrate material must be able to withstand high temperatures without causing the carbon to dissolve. The resulting diamond is often brown, which is a result of inclusions, either carbon or non-diamond.
High growth rate
The growth rate of single-crystal CVD diamonds has been subject to intense research in recent years. Recent studies have revealed that the process can be optimized for increasing growth rates. In addition to this, oxygen is added to the CVD stage to reduce the levels of hydrogen and silicon impurities. This results in a lower growth rate of approximately 10 mm per hour, which is much higher than that of standard processes, which typically only achieve 0.3 mm per hour.
In the present invention, the high-pressure MPCVD process is used to produce single-crystal diamonds with a high growth rate. The process can achieve growth rates of up to 200 mm/hr, depending on the methane concentration and stage design. In addition, the invention is applicable to enlarging anvils and compressing large samples at high pressures.
The process is based on breaking the molecular hydrogen into atomic hydrogen. The catalyst for this conversion can be an intense plasma or hot filament. The gas is applied to the graphite substrate, selectively etches the surface, breaking up double bonds and resulting in the creation of diamonds. This process has been successful in the past for creating diamonds with a high growth rate.
Another advancement in CVD technology is the use of polycrystalline diamonds as electrode materials. This technology allows scientists to study redox reactions that conventional electrodes cannot handle. For example, chemistry professor Greg M. Swain uses CVD to grow polycrystalline boron-doped diamond electrodes to monitor redox-reactive organic contaminants in drinking water. Another application of CVD diamonds involves the development of highly conductive boron-doped single-crystal diamond microelectrodes for use in high-powered CO2 lasers.
ACVD diamond has the same optical properties of a natural diamond. However, unlike a natural diamond, a CVD one can be produced much faster and more cheaply. As a result, CVD diamonds can be sold at a much lower price than their mined counterparts. The process does not create mineral waste and is not associated with any ethical issues. In addition, the production of CVD goods can be more cost effective, allowing the consumer to save 20 to 30% on their diamond purchases.
Fluorescence
One way to understand the origin of CVD single crystal diamonds is to study the processes involved in their formation. A diamond seed of the (100) orientation undergoes a CVD process in six layers, increasing in nitrogen concentration during each layer. Raman spectroscopy and optical absorption are used to measure the effectiveness of nitrogen doping. Birefringence imaging is also used to analyze the absorption spectrum.
One method to measure the NV centers in a CVD diamond is to examine its Raman spectra. The NV centers are more likely to be mobile at higher temperatures than at lower temperatures. The same test can be performed on a CVD diamond of the same spectral range but different temperature. This method allows researchers to measure the NV centers in a diamond’s structure.
The failure mode of a CVD single crystal diamond is cleavage on the 111 plane. In a study by Houwman (2003), he measured the strength of 14 CVD single crystal diamond plates and 14 natural type IIa samples. Both of the materials were polished to a surface roughness of 1 nm. The CVD single crystal diamond plates achieved 5.1 GPa of strength, while the natural type IIa diamonds measured 4.5 GPa.
The CVD diamond’s high sensitivity to radiation is based on its unique property. It has high electron-hole pair creation energy and a low intrinsic carrier fraction. This property makes it a very useful material for measuring various optical properties of materials. A CVD diamond can also demonstrate the ability to produce nanometer-sized objects that have a low intrinsic carrier fraction. In this way, it can show high sensitivity to radiation.
High-quality single crystal material is required for producing CVD diamonds. In addition to its high purity and low impurity content, MPCVD allows for large anvils. High-purity, multicarat single crystal diamonds are produced by MPCVD. Various measurements reveal that these diamonds have high optical quality without layers. High-pressure optical windows require a large intensity ratio between the second-order Raman peak and the fluorescence background.
Impurities
GIA scientists have analyzed a number of samples of CVD single-crystal diamonds, including some with unusual colours. The majority of these samples were colorless, with only a few showing “pink” or “yellow” hues. Interestingly, while natural diamonds can have a range of colours, CVD samples have relatively high levels of impurities and other factors.
Spectroscopic techniques can be used to identify these diamonds, as many CVD synthetics show no reaction to standard long-wave UV light. Gemologists use these methods to verify the quality of synthetic diamonds, because they do not show any of the visual properties of natural diamonds. Fortunately, diamond scientists have developed the technology to identify CVD synthetics with the aid of spectroscopic analysis.
The bulk properties of a diamond are dominated by its grains and grain boundaries, while in a single-crystal diamond, there are point defects and extended defects. Extended defects include twins, vacancy clusters, and dislocations. The latter defects act as electron acceptors or donors in diamonds. CVD diamonds also contain non-carbon atoms, such as Si, P, and S.
Vacancies are one known cause of the brown colour of CVD diamonds. Because the CVD process requires a high growth rate, small voids are left behind. The high growth temperature allows incorporated vacancies to become mobile and re-organize themselves into vacancy disks. The result is a significant energy reduction. In the end, a CVD diamond will have a light brown hue despite its relatively high level of impurities.
Optical properties of CVD single-crystal diamonds can also be improved by minimizing the amount of impurities present in the stone. By controlling the density of impurities, this type of diamond will display an improved optical property, notably low birefringence. It will also enhance the performance of doped dielectric lasers and semiconductor disk lasers.
As-grown CVD samples have a light pink hue, these samples may contain a higher concentration of silicon than those with a low density of silicon. Their fluorescence color depends on the relative concentration of NV0 and NV-centers. These values are important because NV0 and NV-centers can be very easily distinguished in a diamond’s optical property by their corresponding ZPLs.
Conflict-free nature
The light brown color of CVD diamonds is due to a phenomenon called “strain-induced birefringence,” which occurs when the crystal lattices of the two parent crystals do not match. When this happens, the resulting diamond has a “fuzzy” appearance, with numerous train lines that are not considered while grading its clarity. In fact, half of the IGI-graded CVD diamonds have undergone this treatment.
The process used to make CVD diamonds is a relatively new development in the manufacturing process. The diamonds are created by vapor deposition, and the diamonds that are produced undergo several processes that create the stone’s color. One method is called CVD, and involves a high-pressure and high-temperature process. Because the process is relatively new, a number of diamonds are being produced at high pressures and temperatures.
Unlike natural diamonds, CVD single crystal diamonds are not mined in conflict zones. The FTC warns against false claims of conflict-free or sustainable diamonds. While some diamond manufacturers are actively working to ensure better safety, other diamond production processes use huge amounts of energy and chemical vapors. This makes it possible for top-tier players to enter the market with low prices, causing prices to stabilize.
In this process, a diamond seed is introduced into the diamond matrix. The seed material can be a piece of diamond, silicon carbide, molybdenum, quartz glass, or cemented carbide. The substrate material must be able to withstand high temperatures, while the deposited carbon remains in place. Some diamonds are brown because they contain carbon and other non-diamond inclusions.
The optical center of a CVD synthetic diamond has a doublet ZPL at 737 nm. It is related to the presence of the Si-V defect. Moreover, the doublet absorption peak is not typical for a CVD synthetic diamond. Despite the difference between natural and lab diamonds, CVD synthetic diamonds are often more affordable than natural diamonds.
8mm*8mm CVD Single Crystal Diamonds Lab Created Light Brown